Unit 1: Structures, trends, chemical reactions, quantitative chemistry and analysis
Atomic structure
Revise how scientists first viewed the atom, the electronic configuration of an atom and the chemical reactions based on different configurations.
Bonding
Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams.
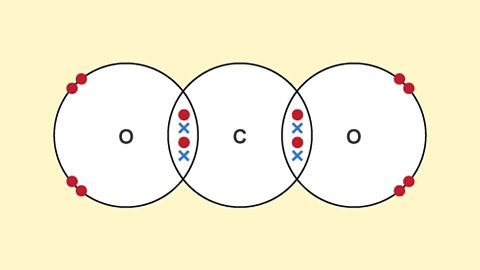
Structures
Revise covalent and ionic compounds and structures, and see how the two types of bond give rise to different physical properties in the resulting substance.

Nanoparticles
Learn about nanoparticle properties and their surface area to volume ratio.
Symbols, formulae and equations
Learn how symbols, formulae and equations help chemists to explain chemical reactions in detail.
The periodic table
Revise the history, patterns and structure of the periodic table including; groups and periods; group 1 metals; transition metals; non-metals; reactivity.

Quantitative chemistry (1)
Learn about how chemists use relative atomic masses and relative formula masses to carry out mole calculations.

Acids, bases and salts
Learn about how many chemicals are acidic, neutral or alkaline and how we can distinguish one from another using indicators.

Chemical analysis
Learn how to separate and analyse chemicals using filtration; crystallisation; paper chromatography; simple and fractional distillation; flame tests.

Solubility
Learn about how solubility is a measurement of the maximum mass of a substance which will dissolve in 100 g of water at a particular temperature.

Links
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link