What are the key learning points about quantitative chemistry (1)?
atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons. are very light, so chemists measure their mass relatively, which means they are compared to the mass of a carbon-12 atom.
A mole is a measurement of a ‘batch’ of particles. It is easier to describe the number of moles of a substance rather than the number of atoms as atoms are very small.
An empirical formula is the simplest ratioA ratio is a way to show the relationship between amounts of one substance compared to another. It is usually written in the form a:b. of the elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. An element cannot be broken down into anything simpler by chemical means. in a compound, and can be worked out by calculation.
Water of crystallisation is water chemically bonded in a crystal structure. The degree of hydration of a compoundA substance formed when two or more elements are chemically combined. can be worked out by calculation.
What are relative masses?
Relative atomic mass
Atoms have a very small mass, so they are difficult to measure accurately.
Instead, chemists use a scale.
On this scale, the mass of a 12C atom is exactly 12.
This scale is convenient as the mass of the atom is the same as the mass numberThe total number of protons and neutrons found in the nucleus of an atom. of the atom.
For example:
- 24Mg has a relative mass of 24 – it is twice as heavy as a 12C atom.
- 1H has a relative mass of 1 – it is 12 times lighter than a 12C atom.
These masses are called relative atomic masses.
They use the symbol Ar or RAM.
The relative atomic mass is the mass of an atom compared with that of the 12C isotopeAtoms which have the same number of protons (so they are atoms of the same element and have the same atomic number) but they have a different number of neutrons (so they have a different mass number)., which has a mass of exactly 12.
Relative atomic mass is the weighted mean mass of the isotopes of an element.
For example, chlorine has two isotopes (35Cl and 37Cl) and the relative atomic mass of chlorine is 35.5.
Relative atomic masses do not have units.
Relative formula mass
The relative formula mass of a substance is the sum of the relative atomic massThe mass of the atom compared with that of the carbon-12 isotope, which has a mass of exactly 12. These masses use the symbol Ar or RAM. of the atoms in the formulaePlural of formula..
Relative formula mass has the symbol, Mr or RFM.
Like Ar values, Mr values also have no units.
To calculate Mr for a substance:
- work out how many atoms of each element are in the chemical formula
- add together the Ar values for all the atoms of each element
For example, the formula for carbon dioxide is CO2.
It contains one carbon atom and two oxygen atoms.
Using the mass numbers from the periodic table, the Ar of carbon is 12, and the Ar of oxygen is 16.
Mr of CO2 = 12 + 16 + 16 = 44
Question
Calculate the relative formula mass, Mr, of calcium hydroxide, Ca(OH)2.
(Ar of Ca = 40, Arof O = 16, Arof H = 1)
Answer
Ca(OH)2 contains: 1 x Ca, 2 x O, 2 x H.
Mr = 40 + (2 × 16) + (2 × 1)
= 40 + 32 + 2
= 74
How to find the percentage of an element in a compound by mass
To find what percentage of the mass of a compound comes from a particular element, you must first calculate the relative formula massThe sum of the relative atomic masses of the atoms in a formula. Relative formula mass has the symbol, Mr or RFM. (Mr) of the compound.
Then, use the following equation:
\({\%~of~an~element} = \frac{mass~of~an~element~in~the~compound}{relative~formula~mass~M_r} \times 100\)
Example
Calculate the percentage by mass of hydrogen in ethanol C2H5OH.
(Ar of C = 12, Ar of H = 1, Ar of O = 16)
Answer
Mr of C2H5OH = (2 × 12) + (5 × 1) + 16 + 1 = 46
There are six atoms of hydrogen in the compound, so the mass of hydrogen is 6 × 1 = 6.
\({\%~of~hydrogen} = \frac{mass~of~hydrogen}{relative~formula~mass~M_r} \times 100 = \frac{6}{46} \times 100 = 13\% \)
Question
Calculate the percentage by mass of oxygen in sodium carbonate, Na2CO3.
(Ar of Na = 23, Ar of C = 12, Ar of O = 16)
Answer
Mr of Na2CO3 = (2 × 23) + 12 + (3 × 16) = 106
There are three atoms of oxygen in the compound, so the mass of oxygen is 3 × 16 = 48.
\({\%~of~oxygen} = \frac{mass~of~hydrogen}{relative~formula~mass~M_r} \times 100 = \frac{48}{106} \times 100 = 45\%\)
What is a mole?
Atoms and molecules are too small to count individually.
Instead, chemists use a quantity called amount of substance, measured in a unit called the mole (mol).
A mole is like a ‘batch’ of atoms, and they are easier to count than individual atoms.
The mass of one mole of any substance is numerically equal to its relative formula mass or relative atomic mass.
For example, the relative atomic mass of carbon is 12.
If you weighed out 12 g of carbon, you would have one mole of carbon atoms.
What is the relationship between moles and masses?
Moles, mass and relative formula mass are closely related.
\(moles = \frac{mass~(g)}{M_r}\)
You can imagine these properties of a substance in a triangle.
You can reconfigure the triangle to calculate a substance’s mass, moles or Mr.
Cover the value you want to find and perform the operation between the remaining two values
How to calculate amounts in moles
Example
Calculate the number of moles in 36 g of water (Mr of water = 18).
\(moles = \frac{mass~(g)}{M_r}\)
\(moles = \frac{36}{18}\)
= 2 mol
How to calculate mass
Example
Calculate the mass of 0.25 mol of carbon dioxide molecules (Mr of CO2 = 44).
Mass = mol × Mr
= 0.25 × 44
= 11 g
How to calculate amounts in moles.
What are industrial quantities? (Higher tier only)
Most chemical processes take place in factories.
Substances are measured out in tonnes and kilograms (kg), rather than in grams.
When calculating moles the mass must always be expressed in grams.
Here is how to convert them:
1 tonne = 1000 kg
1 kg = 1000 g
Example
Calculate the number of moles in 12 kg of magnesium (Ar of magnesium = 24).
Mass of magnesium = 12 kg = 12,000 g
\(moles = \frac{mass~(g)}{M_r}\)
\(moles = \frac{12000}{24}\)
= 500 mol
Question
Calculate the missing values in the table below:
| Substance | Mr | Mass (g) | Moles |
|---|---|---|---|
| NH3 | 17 | 68 | |
| CaCO3 | 100 | 2.5 | |
| MgO | 40 | 120 | |
| CuSO4 | 160 | 0.5 |
Answer
| NH3 | 17 | 68 | \(\frac{68}{17}=4\) |
| CaCO3 | 100 | 2.5 × 100 = 250 | 2.5 |
| MgO | 40 | 120 | \(\frac{120}{4}=3\) |
| CuSO4 | 160 | 0.5 × 160 = 80 | 0.5 |
What is a molar ratio in an equation?
A ratio is a way to show the relationship between the amount of one substance compared to another.
In balanced equations, the number in front of each formulaA formula is made up of the symbols for one or more element and a subscripted number to show how many atoms of each element are bonded together. shows the ratio of the reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. and productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). .
It tells us how many moles of each chemical we need to react to make the products.
If there is no number, the amount is one mole.
Example:
Zn + 2HCl → ZnCl2 + H2
The molar ratio is:
1 mol Zn : 2 mol HCl : 1 mol ZnCl2 : 1 mol H2.
In other words, one mole of Zn will react with two moles of HCl. This will produce 1 mole of ZnCl2 and one mole of H2.
How to calculate masses from equations
balanced chemical equationA chemical equation written using the symbols and formulae of the reactants and products, so that the number of units of each element present is the same on both sides of the arrow. and relative formula massThe sum of the relative atomic masses of the atoms in a formula. Relative formula mass has the symbol, Mr or RFM. can be used to calculate the mass of product made from a given mass of reactant, and vice versa (higher tier only).
Example:
N2 + O2 → 2NO
What mass of nitrogen is needed to make 120 g of nitrogen monoxide?
(Mr of NO = 30, Mr of N2 = 28)
A table layout can be helpful to work out the answer:
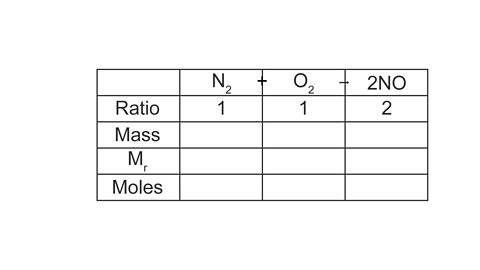
Image caption, 1. Give each substance its own column in the table, and record the ratio using the balancing numbers in the equation.
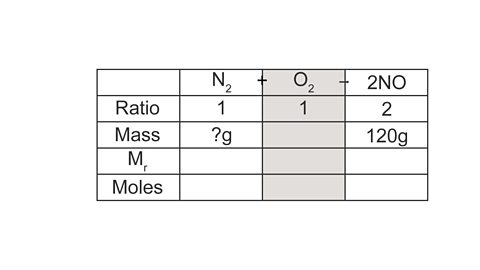
Image caption, 2. Use the information in the question to record the mass given (NO), and the mass you need to calculate (N₂). As O₂ is not mentioned in the question we can ignore its column.
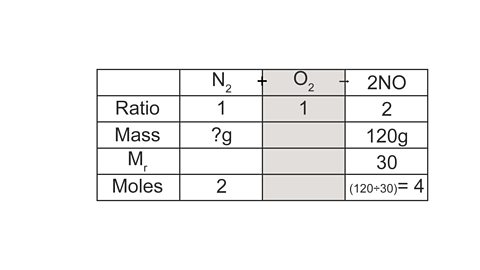
Image caption, 3. Starting with the mass you have been given (for NO), calculate the number of moles.
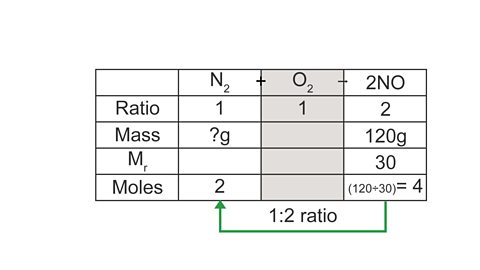
Image caption, 4. Using the ratio, calculate the missing moles value.
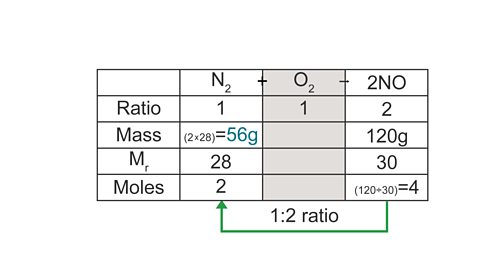
Image caption, 5. Use the number of moles to calculate the missing mass, which is the answer to the question.
1 of 5
Question
2Mg + O2 → 2MgO
Calculate the mass of magnesium oxide that could be produced from 48 g of magnesium.
(Ar of Mg = 24, Mr of MgO = 40)
Answer
Mass of magnesium oxide = 80 g
What are limiting reactants? (Higher tier only)
A reaction finishes when one of the reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. is all used up.
The other reactant has nothing left to react with, so some of it is left over:
- the reactant that is all used up is called the limiting reactant - it sets a limit on how much productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). can form.
- the reactant that is left over is described as being in excess.
The mass of product formed in a reaction depends upon the mass of the limiting reactant.
This is because no more product can form when the limiting reactant is all used up.
Example
2K + S → K2S
What mass of potassium sulfide is formed when 58.5 g of potassium is reacted with 32 g of sulfur?
Again, a table can be useful to structure this calculation:
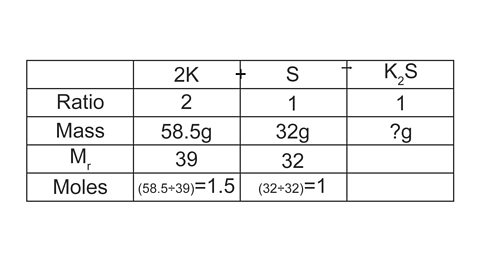
Image caption, 1. Start by calculating the number of moles of each reactant.
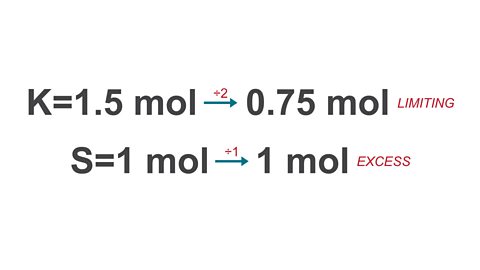
Image caption, 2. To work out which reactant is limiting, divide both mole values by their ratio number. The value that is now the smallest is the limiting reactant. The excess reactant can now be ignored.
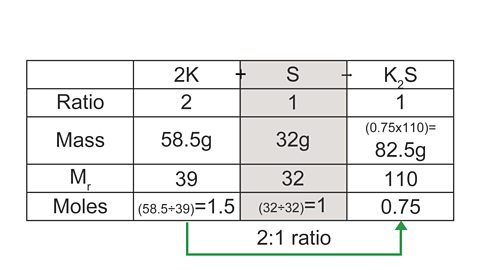
Image caption, 3. Use the moles of the limiting reactant to calculate the moles of the product, and use this value to calculate the final mass.
1 of 3
What is theoretical, actual and percentage yield?
Yield describes the amount of a product that is made in a chemical reaction.
There are three types of yield in chemistry:
Theoretical yield: the maximum possible mass of a product that a chemical reaction can make. It is calculated using molar massThe mass of one mole of a substance. The molar mass is the same as the relative formula mass in grams, or the relative atomic mass in grams. ratioA ratio is a way to show the relationship between amounts of one substance compared to another. It is usually written in the form a:b..
Actual yield: the mass of a product that a chemical reaction makes in real life. It is usually less than the theoretical yield, for a number of reasons:
- some of the product may be lost when the products are removed from the reaction mixture.
- there might be side reactions – unwanted reactions that compete with the desired one.
- the reactions may be reversible and may not go to completion.
Percentage yield: a comparison between actual yield and theoretical yield.
\({percentage~yield } = \frac{actual~yield}{theoretical~yield} \times 100\)
The percentage yield can vary from 100% (no product lost) to 0% (no product made).
Example
12.4 g of copper(II) carbonate are heated and it decomposes.
6 g of copper(II) oxide is formed.
Calculate the percentage yield.
CuCO3 → CuO + CO2
Calculate the theoretical yield, using the same steps as a reacting mass calculation.
theoretical yieldThe maximum possible mass of a product that a chemical reaction can make. It is calculated using molar ratios. of copper(II) oxide = 8 g
actual yieldThe amount of a product made in a chemical reaction. of copper(II) oxide = 6 g
\({Percentage~yield} = \frac{actual~yield}{theoretical~yield} \times 100 = \frac{6}{8} \times 100 = 75\%\)
What is empirical formula and molecular formula?
The molecular formula shows the actual number of atoms of each elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. An element cannot be broken down into anything simpler by chemical means. present in a compound.
The empirical formula of a compoundA substance formed when two or more elements are chemically combined. is the simplest, whole number ratio of atoms of each element in a compound.
A molecular formula can be converted into an empirical formula by simplifying the ratio of the elements in the formula
Example
| Molecular formula | Empirical formula |
|---|---|
| C6H12 | CH2 |
| C6H6 | CH |
| CH4 | CH4 |
How to convert empirical formulae to molecular formulae
You can work out the molecular formulaThe actual number of atoms of each element present in a compound. from the empirical formulaEmpirical formula of a compound is the simplest, whole number ratio of atoms of each element in a compound., if you know the relative formula massThe sum of the relative atomic masses of the atoms in a formula. Relative formula mass has the symbol, Mr or RFM. (Mr) of the compound.
Add up the atomic masses of the atoms in the empirical formula.
Example:
The empirical formula of a hydrocarbon is CH2 and its Mr is 42.
- the mass of the atoms in the empirical formula is 14
- 42 ÷ 14 = 3
- multiply the numbers in the empirical formula by 3
The molecular formula of the hydrocarbon is C3H6.
How to calculate the empirical formula of a compound (higher tier only)
The empirical formula of a compound can be calculated from mass changes that take place during a chemical reaction.
For example, a metal will increase in mass when it reacts with oxygen to produce a metal oxide.
This can be carried out in the apparatus below:
In this experiment the metal would be heated and then weighed a number of times until two consecutive mass readings are the same.
This is known as heating to constant mass.
Example
A sample of titanium was heated in a crucibleA small porcelain dish that can be heated very strongly over a Bunsen burner when it is supported in a pipeclay triangle. to produce titanium oxide.
The following mass data was obtained.
Calculate the empirical formula of titanium oxide.
| Mass of titanium before heating | Mass of titanium oxide after heating to constant mass |
|---|---|
| 9.6 g | 16.0 g |
This calculation is best laid out in a table.
The mass of oxygen is obtained by subtracting the initial mass from the final mass.
This is because it is the addition of oxygen that causes the titanium to increase in mass.
| Ti | O | |
|---|---|---|
| Mass | 9.6 g | (16.0 - 9.6) 6.4 g |
| Ar | 48 | 16 |
| Moles | (9.6 ÷ 48) = 0.2 mol | (6.4 ÷ 16) = 0.4 mol |
| Ratio | (0.2 ÷ 0.2) = 1 | (0.4 ÷ 0.2) = 2 |
| Formula | TiO2 | |
The method involves working out the number of moleA mole of a substance is its relative atomic mass in grams. of both elements.
To convert the number of moles into a whole-number ratio for the empirical formulaEmpirical formula of a compound is the simplest, whole number ratio of atoms of each element in a compound., both values should be divided by the smaller value.
Question
29.5 g of nickel (Ni) was heated in a crucible to obtain nickel oxide.
The mass of nickel oxide obtained after heating to constant mass was 37.5 g.
Calculate the empirical formula of nickel oxide.
Ar of Ni = 59, Ar of O = 16.
Answer
Mass of nickel = 29.5 g
Mass of oxygen = 37.5 - 29.5 = 8 g
| Ni | O | |
|---|---|---|
| Mass | 29.5 g | 8 g |
| Ar | 59 | 16 |
| Moles | (29.5 ÷ 59) = 0.5 mol | (8 ÷ 16) = 0.5 mol |
| Ratio | (0.5 ÷ 0.5) = 1 | (0.5 ÷ 0.5) = 1 |
| Formula | NiO | |
The empirical formula of nickel oxide is NiO.
What is water of crystallisation?
Water of crystallisation is water that is chemically bonded into a crystal structure.
This is found in many ionic compoundAn ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom that has lost an electron)..
Here is the formula for hydrated copper(II) sulfate.
Notice that water of crystallisation is separated from the main formula by a dot.
CuSO4.5H2O ← water of crystallisation
- hydrated means that the solid crystals contain water of crystallisation.
- an anhydrous substance contains no water of crystallisation.
- dehydration is the removal of water of crystallisation.
- the degree of hydration is the number of moles of water of crystallisation chemically bonded in 1 mole of the compound. The degree of hydration in the example above is 5.
How to determine the degree of hydration experimentally
A hydratedThis means that solid crystals contain water of crystallisation. compound loses water of crystallisationWater that is chemically bonded into a crystal structure. when it is heated.
As it loses water of crystallisation, it loses mass.
When it has lost all of its water of crystallisation it is anhydrousA substance containing no water of crystallisation..
We can use difference in the mass between the hydrated and anhydrous compound to calculate the mass of water of crystallisation removed by heating.
The compound should be heated to constant mass to ensure all of the water of crystallisation is removed.
The following apparatus is used:
A common calculation is to work out the value for the degree of hydration in a compound (often shown as ‘x’, or ‘n’).
Example
The following measurements were taken when a sample of hydrated aluminium nitrate Al(NO3)3.xH2O was heated to a constant mass in an oven.
Mass of evaporating basin = 52.67 g.
Mass of evaporating basin + hydrated salt = 56.42 g.
Mass of evaporating basin and contents after heating to constant mass = 54.80 g.
Use the figures above to calculate the degree of hydration in Al(NO3)3·xH2O.
Mass of anhydrous salt = 54.80 – 52.67 = 2.13 g
Mass of water lost = 56.42 – 54.80 = 1.62 g
| Al(NO3)3 | H2O | |
|---|---|---|
| Mass | 2.13 g | 1.62 g |
| Mr | 213 | 18 |
| Moles | (2.13 ÷ 213) = 0.01 mol | (1.62 ÷ 18) = 0.09 mol |
| Ratio | 0.01 ÷ 0.01 = 1 | 0.09 ÷ 0.01 = 9 |
| Formula | Al(NO3)3.9H2O | |
The value of ‘x’ = 9.
Question
A sample of hydrated calcium sulfate (CaSO4.xH2O) was heated to constant mass.
The mass of the solid before heating was 68.8 g and the mass after heating was 54.4 g.
Calculate the degree of hydration of the compound.
(Ar of Ca = 40, Ar of S = 32, Ar of O = 16, Ar of H = 1)
Answer
Mass of CaSO4 = 54.4 g
Mass of H2O = 68.8 – 54.4 = 14.4 g
| CaSO4 | H2O | |
|---|---|---|
| Mass | 54.4 g | 14.4 g |
| Mr | 136 | 18 |
| Moles | (54.4 ÷ 136) = 0.4 mol | (14.4 ÷ 18) = 0.8 mol |
| Ratio | 0.4 ÷ 0.4 = 1 | 0.8 ÷ 0.4 = 2 |
| Formula | CaSO4.2H2O | |
Degree of hydration ‘x’ = 2.
Practical C1: Determine the mass of water in hydrated crystals
Please use the link below to access the article on: Practical C1: Determine the mass of water in hydrated crystals
How to calculate the percentage of water of crystallisation
Once we have the relative formula massThe sum of the relative atomic masses of the atoms in a formula. Relative formula mass has the symbol, Mr or RFM. of a hydrated compound, we can determine how much of this mass is water of crystallisation.
The mass of the water of crystallisation can be worked out by multiplying the Mr of water by the degree of hydration.
Multiply the degree of hydration by the Mr of water, and divide this by the Mr of the whole compound.
Example
Calculate the percentage of water of crystallisation in hydrated copper (II) sulfate, CuSO4.5H2O% of water crystallisation
\({\%~water~of~crystallisation}\)
\( = \frac {degree~of~hydration \times M_r~of~water}{M_r~of~compound} \times 100 = \frac {5 \times 18}{250} \times 100 = 36\%\)
Note: the mass of the water of crystallisation should be included when calculating the Mr of the compound.
How much do you know about quantitative chemistry (1)
More on Unit 1: Structures, trends, chemical reactions, quantitative chemistry and analysis
Find out more by working through a topic
- count8 of 10

- count9 of 10

- count10 of 10

- count1 of 10