What are the key learning points about solubility?
Solubility is a measurement of the maximum mass of a substance which will dissolve in 100 g of water at a particular temperature.
The solubility of solids increases with temperature, while the solubility of gases decreases with temperature.
When a saturated solution is cooled down, some of the soluteThe solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. For example, the main solute in sea water is sodium chloride. in the solution will be deposited at the bottom of the container.
What is solubility?
Some solids can dissolve in water (soluble), while others cannot (insoluble).
For soluble substances there is a wide range in the amount of a solid that can be dissolved in water.
This concept is known as solubility.
For example, for a substance that dissolves easily in water it will take a large amount of the solid to be added to the water before it becomes saturated.
This indicates a high solubility.
Solubility is defined as the mass of a solid required to saturate 100 g of water at a given temperature.
Solubility is measured in grams of a solute per 100 g of water.
If the mass of water is not 100 g, you can scale the solubility values up or down.
A saturated solution is one in which no more solid can dissolve in the liquid at a given temperature.
Question
The solubility of sodium chloride is 40 g/100 g water at 20oC.
What mass of sodium chloride could dissolve in 25g of water?
Answer
By ratio:
10 g of sodium chloride could dissolve in 25 g of water at 20oC.
What is the relationship between solubility and temperature?
The solubility of solids increases as temperature increases.
This can be shown on a solubility curve, which is a graph of the solubility of a substance plotted against temperature.
The graph below shows the solubility curves for a range of solids.
How to determine a solubility curve
You can determine the solubility of a solid in water by using the following method:
- Weigh a known mass of a solid.
- Measure 10cm3 of water. (The density of water is 1 g/cm3, so 10cm3 = 10 g)
- Add the solid to the water and heat until the solid dissolves.
- Cool the mixture and record the temperature, in °C, at which crystals begin to form. The mass of solid added is the mass of this substance required to saturate 10 g of water at the temperature recorded. Multiplying the mass of the substance by 10 gives the solubility in g/100 g water.
- Repeat with different masses of solid.
- Plot a graph of the mass of solid in grams per 100 g of water, against the temperature in °C.
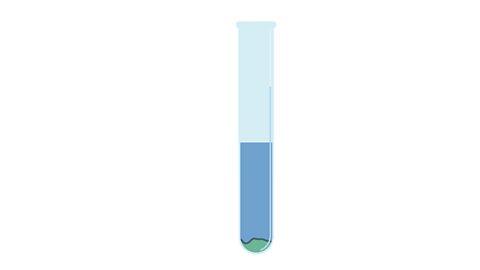
Image caption, 1. In a test tube, a weighed amount of solid is added to 10 g of water. The weighed amount of solid does not fully dissolve in the water.
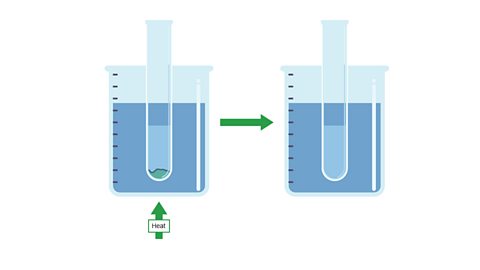
Image caption, 2. The test tube is heated in a hot water bath until the solid completely dissolves.
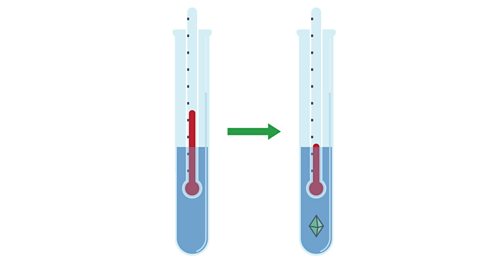
Image caption, 3. The test tube is left to cool and the temperature as the first crystals form is recorded. The mass of solid added is the mass of this substance required to saturate 10 g of water at the temperature recorded.
1 of 3
How to calculate the mass of solid deposited (Higher tier only)
A solid’s solubility decreases with decreasing temperature.
When a hot, concentrated solution is cooled, some of the solute will be deposited.
Example
Calculate the mass of solid that is deposited if a solution containing 15 g per 100 g of water at 30°C is cooled to 10°C.
The solubility of the solid at 30°C is 15 g / 100 g water, and 10 g / 100 g water at 10°C.
Answer
| 30oC | 10oC | ||
|---|---|---|---|
| Solid | Water | Solid | Water |
| 15 g | 100 g | 10 g | 100 g |
At 10oC, the 100 g of water is only able to dissolve 10g of the solid, i.e. it cannot dissolve all 15g.
Any mass of solid beyond the dissolved 10 g will be deposited at the bottom of the container.
Mass of solid deposited = 15 – 10 = 5 g per 100 g of water.
Some harder questions may require scaling of the solubility information.
Question
A solid X, has a solubility of 30 g/100 g water at 50oC, and 20 g/100 g water at 40oC.
Calculate the mass of solid deposited if a saturated solution containing 10 g of water is cooled from 50oC to 40oC.
Answer
Mass deposited = 3 – 2 = 1g.
Some questions require you to use solubility curves to obtain the solubility data required for the question.
Question
Use the graph below to calculate the mass of sodium nitrate, NaNO3, solid that forms when a saturated solution of sodium nitrate, containing 50 g of water, is cooled from 50°C to 10°C.
Answer
From the graph, 50°C the solubility of NaNO3 is 115 g/100 g of water.
At 10°C it is 80 g/100 g of water.
Mass of solid which deposits is 57.5 - 40 = 17.5 g
What is the solubility of gases in water?
While it is more common to think about solids dissolving in water, gases are also able to dissolve in water eg the oxygen dissolved in water allows fish to breathe.
In contrast to solids, the solubility of gases in water decreases as the temperature of the water increases.
If the temperature of sea water rises, less oxygen can dissolve in the water which could harm aquatic life.
How much do you know about solubility?
More on Unit 1: Structures, trends, chemical reactions, quantitative chemistry and analysis
Find out more by working through a topic
- count1 of 10
- count2 of 10
- count3 of 10

- count4 of 10