What are the key learning points about chemical analysis?
A pure substance is a single elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. An element cannot be broken down into anything simpler by chemical means. or compoundA substance formed when two or more elements are chemically combined. not mixed with any other substance.
Impure substances (mixtures) are often useful as carefully designed formulations.
A number of separating techniques can be used to separate mixtures: filtrationMethod used to separate an insoluble solid from a liquid using a physical barrier such as paper., crystallisationThe process of producing crystals from a solution by evaporating the solvent., simple distillationA separation technique which involves a solution being heated so that the solvent evaporates before being cooled to form a pure liquid., fractional distillationThe separation of a mixture of several substances, such as crude oil, by heating; the evaporated components are collected as they condense at different temperatures. or paper chromatographyA separation technique used to separate mixtures of soluble substances. It is used to separate the pigments in a mixture like ink or food colouring. . These techniques can be used to make water safe to drink.
Chemical tests such as flame tests and precipitate tests can be used to identify the different ionElectrically charged particle, formed when an atom gains or loses electrons. in a compound.
What are pure substances?
'Pure' has a specific meaning in chemistry.
A pure substance is a single element or compound not mixed with any other substance.
A mixture contains two or more different substances mixed together, usually easy to separate.
Mixtures are impure.
| Pure substances | Mixtures |
|---|---|
| Diamond only contains the element carbon. | Air is a mixture of oxygen, carbon dioxide, nitrogen and other trace gases. |
| Water only contains the compound water. | Mineral water is a mixture of water and dissolved salts. |
| Table salt only contains the compound sodium chloride. | Milk is a mixture of water, lactose, fat and minerals such as salt. |
Here are some examples of pure substances and mixtures.
What are the melting and boiling points of pure substances and mixtures?
- melting point is the temperature at which a solid changes into a liquid.
- boiling point is the temperature at which a liquid changes into a gas.
Pure substances have specific melting and boiling points.
Impurities cause the following changes to melting and boiling points:
- An impure substance will melt over a range of temperatures and will melt at a lower temperature than expected.
- An impure substance will boil over a range of temperatures and will boil at a higher temperature than expected.
The graphs below show the heating curves for a pure sample of a compound called salol (C13H10O3) and a different sample that contains an impurity.
We can use these differences between melting and boiling points to distinguish between pure substances and mixtures.
Question
The graphs below show the heating curves for two solids.
What is happening at point ‘A’ marked on each graph?
Which substance (X or Y) is pure?
Answer
‘A’ shows the point at which the two substances start to melt.
Substance X is pure as it melts at a sharp, specific melting point.
Substance Y is not pure as it melts over a range of temperatures.
What are formulations?
A formulation is a mixture that has been designed to do something useful.
It is formed by mixing together several different substances in carefully measured quantities to ensure the product has the required properties.
Here are some examples of formulations:
| Formulation | Description | Example |
|---|---|---|
| Alloy | An alloy is a mixture of two or more elements, at least one of which is a metal, and the resulting mixture has metallic properties. An alloy has different properties from the metals it contains. Most alloys are made by melting the metals, mixing them together in liquid form, then leaving them to cool and become solid again. | Stainless steel is an alloy of iron with chromium and other elements e.g. carbon or nickel. |
| Medicine | Medical drugs contain ingredients other than the active drug. These ingredients may help prevent an upset stomach, allow the timed release of the drug, or hold the tablet together. | Calpol® is a formulation of paracetamol and liquid flavourings. |
| fertiliserA substance added to the soil to help plants to grow. | Fertilisers contain nitrogen (N), phosphorus (P) and potassium (K) compounds. The quantities of each vary depending on the plants they are used to fertilise. | NPK fertilisers. |
Formulations are also used in food, fuels, paints and cleaning products.
How are mixtures separated?
The substances in a mixture are relatively easy to separate because they are not chemically joined to each other.
We will look at five different ways of separating mixtures:
| Separating technique | Purpose |
|---|---|
| Filtration | Separating an insolubleAn insoluble substance does not dissolve in water. For example, wax will not dissolve in water. solid from a liquid. |
| Crystallisation | Separating a solubleA solid is soluble if it can dissolve into a specific solvent. For example, salt and sugar are both soluble in water. solid from a solventThe liquid in a solution which dissolves the solute. For example, the solvent in sea water is water.. |
| Paper chromatography | Separating a mixture of soluteThe solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. For example, the main solute in sea water is sodium chloride. in a solution. |
| Simple distillation | Separating a solvent from a solution or separating two miscibleLiquids which can mix together. liquids with different boiling points. |
| Fractional distillation | Separating a mixture of liquids in which the liquids have boiling points that are close together. |
The following terms are useful to know when thinking about separating mixtures.
- a solute is the substance that dissolves in a solvent.
- a solvent is the liquid in which a solute dissolves.
- a solution is a solute dissolved in a solvent.
- a soluble substance is one which will dissolve in a solvent.
- an insoluble substance is one which will not dissolve in a solvent.
- a filtrate is a filtered solution.
- a residue is a solid left behind after filtration.
- a distillate is a liquid produced by distillation.
- miscible liquids are ones that can mix together.
- immiscible liquids are ones that cannot be mixed together.
- evaporation is when a liquid is heated and changes state into a gas.
- condensation is when a gas cools and changes state into a liquid.
What is filtration?
Filtration is used to separate an insoluble solid from a liquid – sand from water for example.
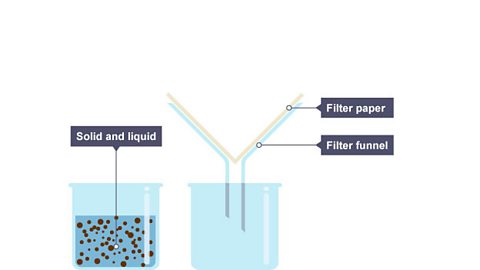
Image caption, 1. One beaker contains a mixture of solid and liquid. The other contains a funnel with filter paper.
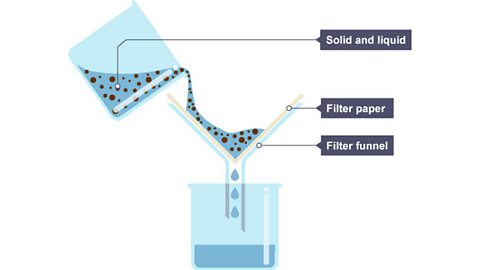
Image caption, 2. The solid and liquid mixture is poured into the filter funnel.
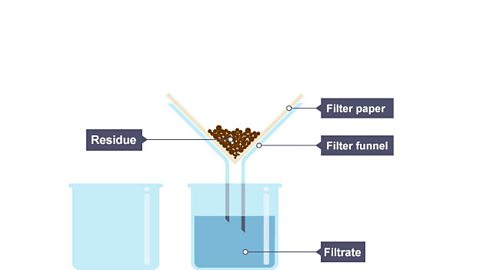
Image caption, 3. The filter paper holds back the solid particles (residue) while allowing the liquid (filtrate) to drip through.
1 of 3
What is crystallisation (evaporation)?
Evaporation is when a liquid is heated and changes state into a gas.
When a solution is heated, some of the solvent evaporates, leaving behind a saturated solution – one in which no more solid can dissolve at that temperature.
The saturated solution is allowed to cool and crystals form.
The crystals can be separated out by filtration.
This process is known as crystallisation.
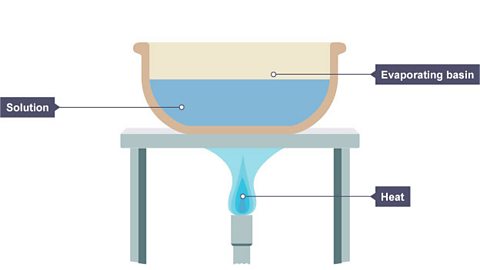
Image caption, 1. A solution is placed in an evaporating basin and heated with a Bunsen burner.
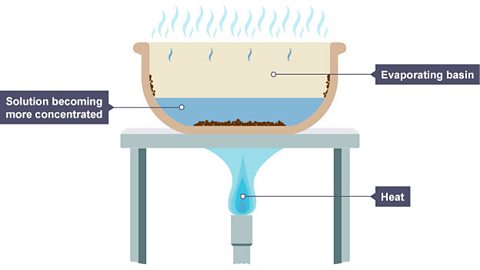
Image caption, 2. The water evaporates, reducing the solution’s volume. Solid particles begin to form in the basin.
1 of 2
What is distillation?
Simple distillation separates a solvent from a solution using both evaporation and condensation – the change of state as gas cools and forms a liquid.
Simple distillation works because the dissolved solute has a much higher boiling point than the solvent.
As the solution is heated, the solvent evaporates.
This gas moves away through the condenser, cools, and condenses and is collected– this liquid is called the distillate.
The remaining solution becomes more concentrated as the amount of solvent decreases.
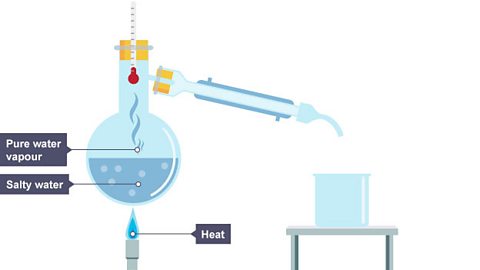
Image caption, 1. Salty water is heated.
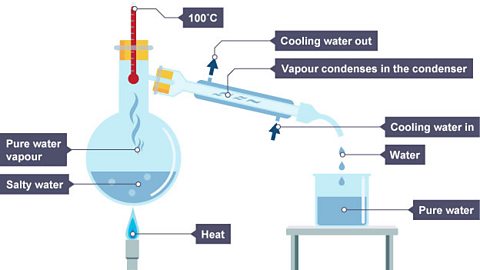
Image caption, 2. The water vapour cools in the condenser and drips into a beaker.
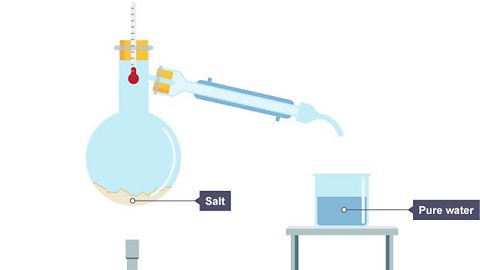
Image caption, 3. The water has condensed as a distillate in the beaker. The salt stays behind.
1 of 3
What is fractional distillation?
Miscible liquids are ones that can mix together – like water and ethanol.
Immiscible liquids are ones that cannot mix together – like oil and water.
Fractional distillation separates miscible liquids that have different boiling points.
It is useful for separating ethanol from a mixture of ethanol and water, and for separating crude oil into different products such as petrol, diesel and kerosene.
When the mixture is heated:
- the liquids boil at their boiling point.
- their vapours rise through a column which is hot at the bottom and cooler at the top.
- each vapour condenses when it reaches a part of the column that is below the temperature of its boiling point.
- the liquids which condense in the column drip back into the flask.
- any gas that makes it to the top of the fractioning column enters the condenser where it is changed to a liquid.
- the liquid runs into a collecting vessel.
- distillates collected over a narrow temperature range are known as fractions.
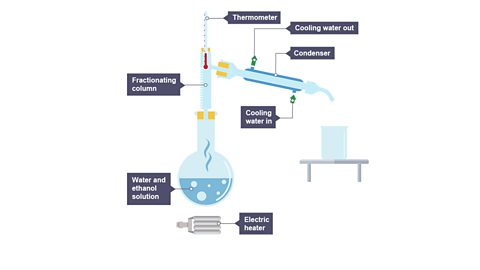
Image caption, 1. Heat the water and ethanol solution.
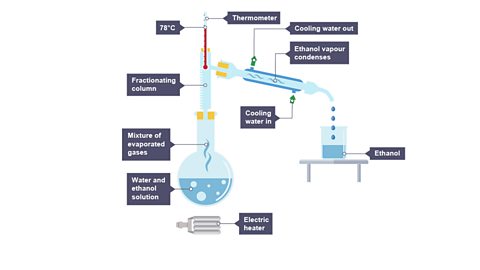
Image caption, 2. The ethanol evaporates first, cools, then condenses.
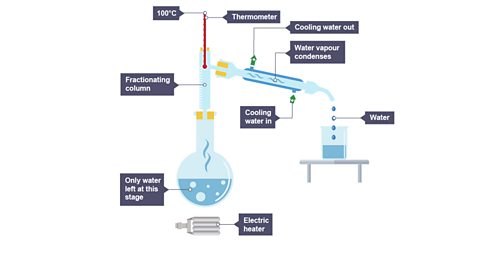
Image caption, 3. The remaining water evaporates, cools and condenses.
1 of 3
What is paper chromatography?
Different substances travel through a piece of chromatography paper at different speeds.
Paper chromatography uses this to separate mixtures of soluble substances.
It provides clues on the possible identity of the substances in the mixture.
These substances are often coloured, such as food colourings, inks, dyes or plant pigments.
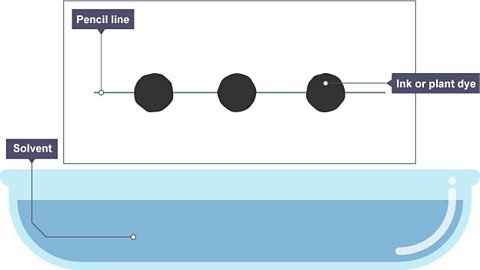
Image caption, 1. Spots of ink or plant dye are placed on a pencil line called the ‘origin’ or ‘baseline’
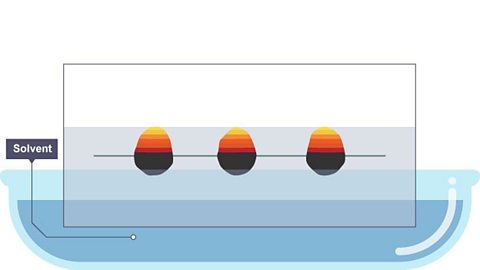
Image caption, 2. Some of the dye spreads up the paper as it is lowered into the solvent.
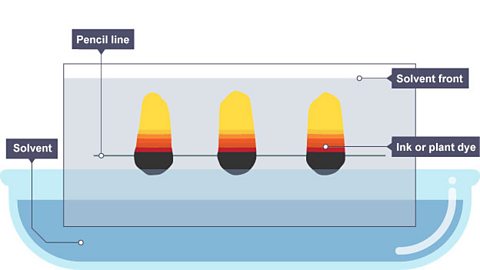
Image caption, 3. The dye spreads up the paper as it absorbs the solvent.
1 of 3
Paper chromatography relies on two phases, each with different properties.
The paper is the stationary phase and the solvent is the mobile phase.
The dissolved substances move at different rates because the strength of their attraction to each phase is different.
Substances that have a strong attraction to the paper move slowly and only travel a short distance.
Substances that have a strong attraction to the solvent move quickly and travel further.
How to interpret a chromatogram
A paper chromatogram can be used to distinguish between pure and impure substances.
- A pure substance produces a single spot on the chromatogram.
- An impure substance produces two or more spots.
The chromatogram can also help to identify substances by comparing them to known substances.
Two substances are likely to be the same if:
- they produce the same number of spots with matching colours.
- the spots travel the same distance up the paper and have the same Rf value (see below).
What is the Rf value?
The Rf value is a measure of the distance the substance travels, relative to the distance travelled by the solvent in the paper.
You can identify an unknown substance by comparing its Rf value to the Rf values of a range of known substances.
Rf=\(\frac{distance~travelled~by~the~substance}{distance~travelled~by~the~solvent}\)
Rf values vary from 0 (the substance is not attracted to the solvent) to 1 (the substance is not attracted to the paper).
How to choose the best method of separation
You will have to choose the best separation technique when you are asked to separate the components of a mixture.
This table can help:
| Mixture | Separating technique |
|---|---|
| Insoluble solid and liquid | Filtration |
| Soluble solid and liquid (solution) | Crystallisation (to obtain solid) or distillation (to obtain liquid) |
| Two miscible liquids | Fractional distillation |
| Soluble solids dissolved in a solvent | Paper chromatography |
Question
The table below describes four examples of mixtures.
Which separation methods could be used to separate the mixtures?
| A | A mixture of food colourings |
| B | Sand and water |
| C | Salt water (to obtain the water) |
| D | Ethanol and water (ethanol is a liquid) |
| E | Salt water (to obtain the salt) |
| A | A mixture of food colourings | Paper chromatography |
| B | Sand and water | Filtration |
| C | Salt water (to obtain the water) | Distillation |
| D | Ethanol and water (ethanol is a liquid) | Fractional distillation |
| E | Salt water (to obtain the salt) | Crystallisation |
What is the process for making water safe to drink?
Potable water is water that is safe to drink.
Water must be treated to make it safe to drink.
How to make potable water from fresh water
Fresh water contains objects – such as branches and leaves, insoluble particles like grit, and harmful microorganisms – that must be removed to make it potable.
Different separation methods and treatments are used to make fresh water potable.
1. Filtration
Filtration removes insoluble solids like stones and leaves.
The water is passed through layers of sand and gravel called filter beds.
2. Sedimentation
Aluminium sulfate is added.
This helps tiny particles to clump together into larger particles.
These settle to the bottom and the clean water can be drawn off the top.
3. Chlorination
Chlorine gas is bubbled through the water to kill any harmful microorganisms, such as bacteria.
How to make potable water from seawater (desalination)
Desalination is the removal of salt from seawater.
This produces clean drinking water and is particularly useful in countries that have coastlines but no readily available fresh water sources, such as rivers and streams.
Desalination is often carried out by distillation:
- the salt water is heated or the water is allowed to evaporate
- the water vapour is collected rather than being lost
- the water vapour is condensed to form pure water/fresh water
- the salt is left behind and can be used for other purposes
Desalination uses a lot more energy than treatment of fresh water and is more expensive.
It is commonly used in the Middle East as rainfall is low, and some countries that are quite wealthy.
Test for water

Pure copper(II) sulfate is white. It is also known as anhydrous copper(II) sulfate because it has no water of crystallisationWater that is chemically bonded into a crystal structure. in it.
When water is added to anhydrous copper(II) sulfate it changes in colour from white to blue.
This colour change can be used as a test for water.
anhydrous copper(II) sulfate + water ⇌ hydrated copper(II) sulfate
CuSO4 + 5H2O ⇌ CuSO4.5H2O
white ⇌ blue
| CuSO4 + 5H2O ⇌ CuSO4.5H2O | |
|---|---|
| white | blue |
Testing for ions
Chemists are often asked to test a substance to find out if it contains specific ionElectrically charged particle, formed when an atom gains or loses electrons..
There are a variety of techniques.
Testing for cations (positive ions)
There are two methods to test for metal cations:
- Flame tests
- Precipitate tests
What are flame tests?
Most metal ions produce a strong colour when put into a blue Bunsen burner flame.
The colour of the flame can be used to identify which metal cations are present in an unknown sample.
Method:
- Take a clean nichrome wire.
- Dip the loop into concentrated hydrochloric acid and then into the sample.
- Hold the nichrome wire in a blue Bunsen burner flame and observe the colour.
Different metal ions produce different coloured flames.
| Ion present | Flame colour |
|---|---|
| Lithium, Li+ | Crimson |
| Sodium, Na+ | Yellow/orange |
| Potassium, K+ | Lilac |
| Calcium, Ca2+ | Brick red |
| Copper, Cu2+ | Blue-green/green-blue |
Precipitate tests
Many tests for anions and cations involve precipitation reactions.
When metal ions combine with the hydroxide ions (OH-) from either sodium hydroxide solution or ammonia solution, they form precipitates (insoluble solids) with characteristic colours.
Method:
- dissolve a small quantity of the unknown substance in water
- place about 5cm3 of the solution into a test tube
- add a few drops of sodium hydroxide solution
- record the colour of any precipitate that is formed
- add dilute sodium hydroxide solution until it is in excess and record the result
You can use ammonia solution instead of sodium hydroxide solution, but there are different results for aluminium and copper(II) salts when you use excess ammonia.
See the table below:
| Metal ion | Result on adding NaOH or NH3 solution | Ionic equation | Effect of adding excess NaOH solution | Effect of adding excess NH3 solution |
|---|---|---|---|---|
| copper(II), Cu2+ | blue precipitate | Cu2+(aq) + 2OH-(aq) → Cu(OH)2(s) | blue precipitate remains | blue precipitate dissolves and a deep blue solution forms |
| iron(II), Fe2+ | green precipitate | Fe2+(aq) + 2OH-(aq) → Fe(OH)2(s) | green precipitate remains | green precipitate remains |
| iron(III), Fe3+ | brown precipitate | Fe3+(aq) + 3OH-(aq) → Fe(OH)3(s) | brown precipitate remains | brown precipitate remains |
| magnesium, Mg2+ | white precipitate | Mg2+(aq) + 2OH-(aq) → Mg(OH)2(s) | white precipitate remains | white precipitate remains |
| aluminium, Al3+ | white precipitate | Al3+(aq) + 3OH-(aq) → Al(OH)3(s) | white precipitate dissolves and a colourless solution forms | white precipitate remains |
| zinc, Zn2+ | white precipitate | Zn2+(aq) + 2OH-(aq) → Zn(OH)2(s) | white precipitate dissolves and a colourless solution forms | white precipitate dissolves and a colourless solution forms |
Tests for anions
Testing for halide ions (Cl-, Br-, I-)
The halogens are the elements in Group 7 of the periodic table, and include chlorine, bromine and iodine.
Their ions are called halide ions e.g. chloride, Cl-.
You can test for them using silver nitrate solution.
Method
- dissolve a small sample of the solid salt you are testing in water.
- place approximately 10cm3 of the solution into a test tube.
- add four drops of nitric acid.
- add silver nitrate solution, dropwise.
- if a precipitate is produced, observe the colour.
Here is a summary of possible results:
| Anion | Colour of the precipitate | Ionic equation |
|---|---|---|
| chloride ion, Cl- | white | Ag+(aq) + Cl-(aq) → AgCl(s) |
| bromide ion, Br- | cream | Ag+(aq) + Br-(aq) → AgBr(s) |
| iodide ion, I- | yellow | Ag+(aq) + I-(aq) → AgI(s) |
The silver ions and the halide ions join together to form a precipitate.
Testing for sulfate ions (SO₄²⁻)
Method:
- dissolve a small sample of the solid salt you are testing in water.
- place about 10cm3 of the solution into a test tube.
- add barium chloride solution, dropwise.
- if a precipitate is produced, observe the colour.
Result
| Anion | Colour of the precipitate | Ionic equation |
|---|---|---|
| sulfate ion, SO42- | white | Ba2+(aq) + SO42-(aq) → BaSO4(s) |
The barium ions and the sulfate ions join together to form a precipitate.
Testing for carbonate ions
Method:
- add dilute hydrochloric acid to the solid salt you are testing
- test any gas that forms by bubbling it through limewater
| Observations |
|---|
| Fizzing |
| Solid dissolves |
| Limewater changes from colourless to milky |
Here is the equation for the reaction between a carbonate compound and hydrochloric acid.
Carbon dioxide is a product of the reaction and will turn limewater milky.
CaCO3 + 2HCl → CaCl2+ CO2 + H2O
Planning experiments to identify ions
You will be asked to plan an experiment to identify the ions present in an unknown compound.
Question
Describe two chemical tests you could carry out to identify the lithium ions and sulfate ions in a solid sample of lithium sulfate.
Answer
Lithium ions
Carry out a flame test: dip a nichrome wire into concentrated hydrochloric acid and then into the sample. Hold the wire in a blue Bunsen burner flame and a crimson flame is observed.
Sulfate ions
Dissolve the sample in a small amount of water. Add a few drops of barium chloride solution and a white precipitate is observed.
Prescribed Practical C4 - Identify the ions in an ionic compound
Please use the link below to access the article on: Prescribed Practical C4 - Identify the ions in an ionic compound using chemical tests
Test your knowledge
More on Unit 1: Structures, trends, chemical reactions, quantitative chemistry and analysis
Find out more by working through a topic
- count10 of 10

- count1 of 10
- count2 of 10
- count3 of 10
