What are the key learning points about energy changes in chemistry?
Exothermic reactions give heat out to the surroundings. Endothermic reactions take heat in from the surroundings.
The energy changes that take place during a reaction can be shown on a reaction profile diagram.
Energy change values can be calculated by comparing the energy required to break the bonds in the reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. , and the energy released by making the bonds in the productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). .
What energy changes happen during reactions?
During a chemical reaction, heat energy can be given out or taken in by the chemicals.
This can cause the temperature of the surroundings to change.
What are exothermic reactions?
An exothermic reaction gives heat out to its surroundings.
The temperature of its surroundings increases.
Exothermic reactions include:
combustionThis is the scientific word for burning. It is where a substance reacts with oxygen from the air and transfers energy to the surroundings by heating and light. reactions.
many oxidationA type of chemical reaction when a substance reacts with oxygen. reactions.
most neutralisationA reaction between an acid and a base producing a salt and water. reactions.
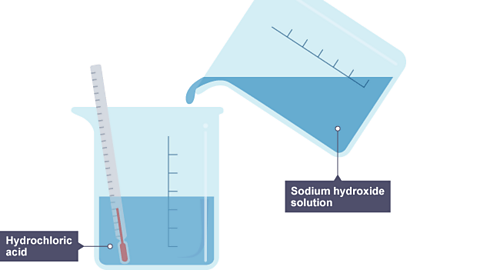
Image caption, 1. Sodium hydroxide solution is poured into a beaker of hydrochloric acid. Inside is a thermometer showing room temperature.
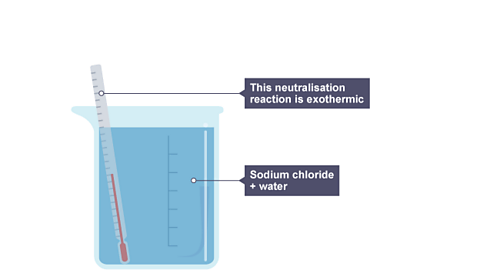
Image caption, 2. The beaker now contains sodium chloride and water. The thermometer shows a rise in temperature. The neutralisation reaction is exothermic.
1 of 2
What are endothermic reactions?
An endothermic reaction takes heat in from its surroundings.
The temperature of its surroundings decreases.
Endothermic reactions include:
thermal decompositionBreaking down a compound into its elements, or simpler compounds using a high temperature. reactions.
the reaction of ethanoic acid and sodium carbonate.
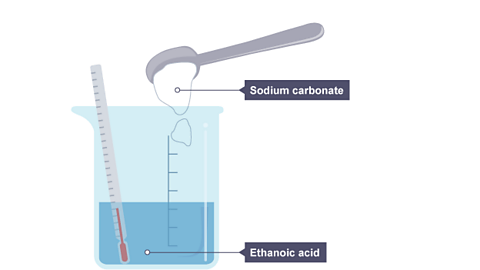
Image caption, 1. Sodium carbonate powder is tipped into a beaker of ethanoic acid, which contains a thermometer showing room temperature.
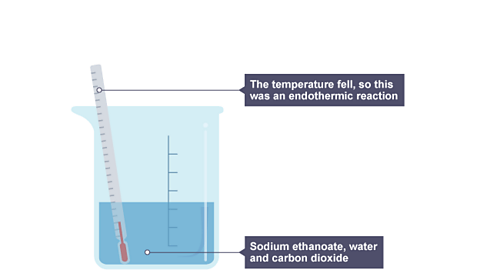
Image caption, 2. The beaker now contains sodium ethanoate, water and carbon dioxide. The thermometer shows a fall in temperature. This is an endothermic reaction.
1 of 2
What are reaction profiles? (Higher tier only)
What are energy level diagrams?
An energy level diagram shows whether a reaction is exothermicA physical change or chemical reaction that transfers energy to the surroundings. or endothermicA physical change or chemical reaction that absorbs energy from the surroundings..
It shows the energy in the reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. and productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). , and the difference in energy between them.
What is an energy level diagram for an exothermic reaction?
In an exothermic reaction heat energy is given out to the surroundings.
This means the chemicals in the reaction will have a lower energy at the end of the reaction.
Since the energy level of the chemicals decreases, exothermic reactions have negative energy change values.
What is an energy level diagram for an endothermic reaction?
In an endothermic reaction heat energy is taken in from the surroundings.
This means the chemicals in the reaction will have a higher energy at the end of the reaction.
Since the energy level of the chemicals increases, endothermic reactions have positive energy change values.
What is activation energy?
A reaction profile is similar to an energy level diagram, but it includes the activation energy – the minimum energy that particles needed for a reaction to occur.
The activation energy is shown by a line called the ‘reaction pathway.’
Activation energy appears as a hump in the line.
The activation energy is equal to the difference in energy between the top of the hump and the energy of the reactants.
The overall change in energy is the difference between the energy of the reactants and products.
Exothermic reaction
Endothermic reaction
How to explain energy changes in reactions
What is making and breaking bonds?
Energy is transferred when chemical bonds are broken or are formed.
Breaking bonds requires energy (endothermic).
Making bonds gives out energy (exothermic).
During a chemical reaction:
bonds in the reactants are broken (endothermic) and
new bonds are made in the products (exothermic).
For example, in the reaction between hydrogen and oxygen (2H2 + O2 → 2H2O)
The difference between the energy needed to break bonds, and the energy released when new bonds are made, determines the type of reaction.
A reaction is exothermic if more heat energy is released in making the bonds in the products, than is absorbed when breaking bonds in the reactants.
A reaction is endothermic if less heat energy is released in making bonds in the products than is absorbed when breaking bonds in the reactants.
Question
The reaction between nitrogen and oxygen to produce nitrogen dioxide is endothermic.
N2 + 2O2 → 2NO2
Explain, in terms of bonds, why this reaction is endothermic.
Answer
Bonds are broken in N2 and O2, and bond breaking requires energy.
Bonds are made in NO2, and bond making gives out energy.
In this reaction more energy is taken in to break bonds than is given out when bonds are made.
How to calculate energy changes
Bond energy
The energy change in a reaction can be calculated using bond energies.
A bond energy is the amount of energy needed to break one moleA mole of a substance is its relative atomic mass in grams. of a particular covalent bondA covalent bond is formed by a shared pair of electrons..
A bond energy value can also tell you the amount of energy that is given out when one mole of a particular covalent bond is formed.
For example, the bond energy of a H-H bond is 436 kJ. This means that:
436 kJ of energy is taken in to break one mole of H-H bonds.
436 kJ of energy is given out when one mole of H-H bonds is formed.
To calculate an energy change for a reaction:
add together the bond energies for all the bonds broken in the reactants.
add together the bond energies for all the bonds formed in the products.
energy change = energy for bonds broken – energy for bonds made.
Exothermic reactions have negative energy change values.
Endothermic reactions have positive energy change values.
Example
Hydrogen and chlorine react to form hydrogen chloride gas:
H−H + Cl−Cl → 2 × (H−Cl)
Use the bond energies in the table to calculate the energy change for this reaction.
| Bond | Bond energy |
|---|---|
| H−H | 436 kJ |
| Cl−Cl | 243 kJ |
| H−Cl | 432 kJ |
Bonds broken (1 × H-H, 1 × Cl-Cl) = 436 + 243 = 679 kJ
Bonds made (2 × H-Cl) = (2 × 432) = 864 kJ
Energy change = bonds broken – bonds made
= 679 - 864
= -185 kJ
The energy change is negative.
This shows that the reaction is exothermic.
Question
Hydrogen bromide decomposes to form hydrogen and bromine:
2 × (H−Br) → H−H + Br−Br
Use the bond energies in the table to calculate the energy change for this reaction.
| Bond | Bond energy |
|---|---|
| H−Br | 366 kJ |
| H−H | 436 kJ |
| Br−Br | 193 kJ |
Answer
Bonds broken (2 × H-Br) = 2 × 366 = 732 kJ
Bonds made (1 × H-H, 1 × Br-Br) = 436 + 193 = 629 kJ
Energy change = bonds broken – bonds made
= 732 - 629
= +103 kJ
The energy change is positive.
This shows that the reaction is endothermic.
How much do you know about energy changes in chemistry?
More on Unit 2: Further chemical reactions, rates and equilibrium, calculations and organic chemistry
Find out more by working through a topic
- count9 of 9

- count1 of 9

- count2 of 9

- count3 of 9
