What are the key learning points about rates of reaction?
The rate of reaction can be determined by measuring the rate of gas production, massThe amount of matter an object contains. Mass is measured in kilograms (kg) or grams (g). loss or precipitateAn insoluble solid which may be formed by mixing two solutions. formation in a chemical reaction.
A rate of reaction is determined by how often successful collisionWhen two objects meet and interact, eg two particles moving towards each other will collide. of particles take place, which are collisions that possess the activation energyThe activation energy is the minimum energy required for a reaction to occur.
The rate of reaction is affected by changing concentrationThe amount of a substance that has been dissolved in a certain amount of solution. Measured in mol/dm3 (moles per decimetre cubed)., temperature, surface area or by using a catalyst A substance that speeds up a chemical reaction without being used up or chemically changed. It does this by providing an alternative reaction pathway with a lower activation energy..
How to measure rates of reaction
Some chemical reactions take place rapidly, while others are very slow.
Chemists compare the speed of different reactions by calculating the rate of reaction.
The rate of reaction can be found by measuring the amount of productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). formed, or the amount of reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. used up, over a certain period of time.
The mass of a solid product is measured in grams (g), while the volume of a gaseous product is measured in cm3.
Rate is most often calculated using the equation: rate = \(\frac {1}{time}\) where the time is the time for the reaction to reach a certain point or the time for the reaction to be completed.
The units of rate calculated in this way are s-1.
What experiments are used to measure the rate of reaction?
The experiment you carry out to measure the rate of reaction depends on the nature of the product being measured.
1. How to measure the amount of gas produced
In reactions that produce a gas, the volume of a gas can be measured with a gas syringe (or an upside down measuring cylinder).
The following method is carried out:
The reactants are mixed together in a conical flask and a gas syringe is connected to the flask.
At the same time, a stop-clock is started.
The scale on the gas syringe is used to record the volume of gas produced at regular time intervals.
A graph of ‘volume of gas produced/ cm3**' against 'time / s' is plotted.
Examples
Examples of reactions that would be suitable for this method are the reactions of magnesium, zinc or calcium carbonate with hydrochloric acid where hydrogen or carbon dioxide gas is produced.
Mg + 2HCl → MgCl2 + H2
Zn + 2HCl → ZnCl2 + H2
CaCO3 + 2HCl → CaCl2 + H2O + CO2
2. How to measure the loss of mass from a reaction mixture
In reactions that produce a gas, an alternative method of measuring the rate of reaction is to measure the mass of the reacting mixture during the reaction.
As gas escapes from the reacting mixture the mass of mixture will decrease over time.
The following method is carried out:
The reactants are mixed together in a conical flask and the flask is placed on a balance.
The initial mass of the reacting mixture is measured, and at the same time a stop-clock is started.
The mass of the reacting mixture is measured at regular time intervals.
A graph of ‘mass lost / g’ against ‘time / s’ is plotted.
The cotton wool prevents any loss of mass caused by liquid spray.
Examples
Examples of reactions that would be suitable for this method are the reactions of magnesium, zinc or calcium carbonate with hydrochloric acid where hydrogen or carbon dioxide gas is produced.
Mg + 2HCl → MgCl2 + H2
Zn + 2HCl → ZnCl2 + H2
CaCO3 + 2HCl → CaCl2 + H2O + CO2
3. How to measure the formation of a precipitate
Sodium thiosulfate solution reacts with dilute hydrochloric acid to produce sodium chloride, water, sulfur dioxide and sulfur.
The sulfur precipitate makes the solution change to opaqueA material, object or substance which does not allow light to pass through it..
The following method is carried out:
The sodium thiosulfate solution is mixed with hydrochloric acid in a conical flask, and a stop-clock is started.
The flask is placed on a piece of paper with an ‘X’ drawn on it.
The stop-clock is stopped when the ‘X’ is no longer visible when viewed from above.
The rate of reaction is calculated using: rate = \(\frac {1}{time}\)
How to analyse rates of reaction data
In a typical rates experiment, the mass or volume of productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). is measured at regular time intervals.
The results are usually recorded in a table.
For example:
| Time / min | Volume of gas produced / cm3 |
|---|---|
| 0 | 0 |
| 1 | 34 |
| 2 | 42 |
| 3 | 48 |
| 4 | 50 |
| 5 | 50 |
These results show that the rate decreased during the reaction as the volume of gas produced each minute decreased as the time increased.
The reaction had finished by four minutes as no more gas was produced after that point.
Graphs
The rate of reaction can be analysed by plotting a graph of ‘volume of gas’ or ‘mass lost’ against time. The graph below shows an example for two reactions.
For both reactions, the graph lines show that:
- The reactions become slower as time goes on (the gradients of the lines decrease).
- The reactions have stopped at the points that the lines become horizontal.
Compared to the slow reaction, the graph line for the faster reaction:
- has a steeper gradient at the start
- becomes horizontal sooner (gas stops being produced sooner so the reaction finishes sooner) meaning that the rate of reaction is greater.
Question
The graph below shows the volume of gas produced in by a reaction over time.
At what time did the reaction finish and what was the total volume of gas produced?
Answer
The reaction finished at 6 minutes, as the graph line becomes horizontal at this point.
This indicates that no more gas is being produced, so the reaction has stopped.
The total volume of gas produced is 60 cm3.
What factors affect the rate of reaction? (Higher tier only)
Collision theory
For a chemical reaction to occur, the reactant particles must collisionWhen two objects meet and interact, eg two particles moving towards each other will collide. with each other.
However, a collision with too little energy will not produce a reaction:
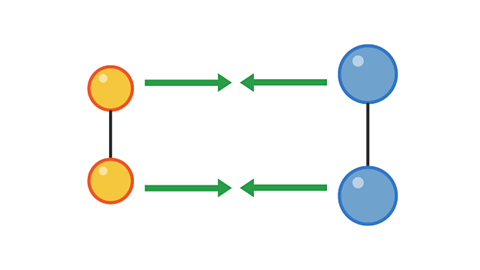
Image caption, 1. Two pairs of particles move towards each other
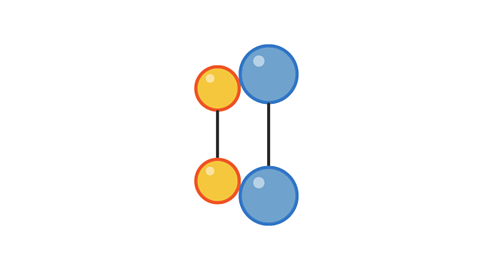
Image caption, 2. The pairs have collided, but not with enough energy to cause a reaction.
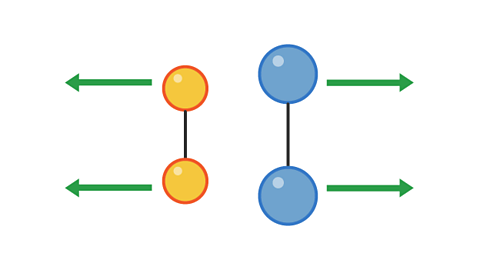
Image caption, 3. The pairs bounce off each other and move apart.
1 of 3
The colliding particles must have enough energy for the collision to be successful in producing a reaction.
The minimum amount of energy for a collision to be successful is called the activation energy.
The rate of a reaction depends on the rate of successful collisions between reactant particles.
The more successful collisions there are, the faster the rate of reaction.
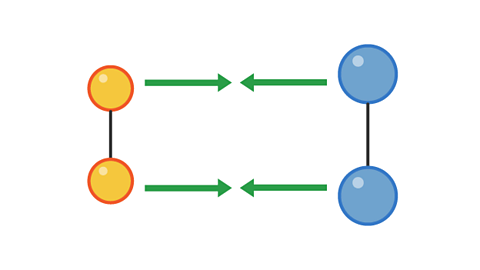
Image caption, 1. Two pairs of particles move towards each other
Image caption, 2. The pairs collide with enough energy to cause a reaction. This is a successful collision.
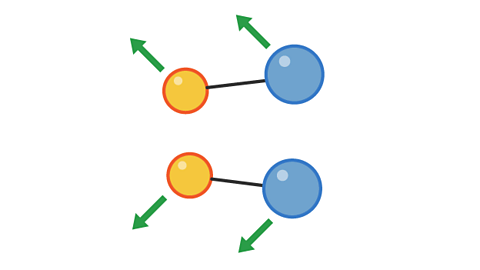
Image caption, 3. The products move away from each other after the collision.
1 of 3
How does temperature affect the rate of reaction?
The rate of a chemical reaction can be changed by altering the temperature. If the temperature is increased:
The reactant particles have more energy.
They move more quickly and collide more often.
There are more successful collisions per unit time; and
the rate of reaction increases.
How does concentration affect the rate of reaction?
The rate of a chemical reaction can be changed by altering the concentration of a reactant in solution. If the concentration is increased:
There are more reactant particles.
They collide more often.
There are more successful collision per unit time; and
the rate of reaction increases.
How does solid particle size (surface area) affect the rate?
The rate of a chemical reaction can be increased by using smaller solid particles which increases the surface area of a solid reactant.
This is done by cutting the substance into small pieces, or by grinding it into a powder. If the surface area of a reactant is increased:
More particles are exposed to the other reactant.
They collide more often.
There are more successful collisions per unit time; and
the rate of reaction increases.
This shows that the rate of reaction is greater when the surface area is increased.
Key fact
‘Per unit time’ is used in place of ‘per second’ or ‘per minute’ when it does not matter which unit of time you are referring to.
What are catalysts?
The rate of a reaction can be increased by adding a suitable catalyst.
A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at the end).
A catalyst speeds up a reaction by providing an alternative reaction pathway of lower activation energy.
There are more successful collisions per unit time; and
the rate of reaction increases.
This alternative reaction pathway can be shown on an energy level diagram.
Catalytic decomposition of hydrogen peroxide
Hydrogen peroxide decomposes to produce water and oxygen gas.
2H2O2 → 2H2O + O2
This reaction is normally very slow, but the rate can be increased by adding a manganese(IV) oxide catalyst.
Manganese(IV) oxide is a black solid and can be removed from the reaction mixture at the end by filtrationMethod used to separate an insoluble solid from a liquid using a physical barrier such as paper..
The rate of this reaction can be investigated by measuring the volume of oxygen gas produced (using a gas syringe) or by measuring the mass lost from the reaction mixture.
How to carry out prescribed practical C6
Please use the link below to access the article on: Prescribed Practical C6 - Investigate how changing a variable changes the rate of reaction
WATCH: Four ways to increase the rate of a reaction
Test your knowledge
More on Unit 2: Further chemical reactions, rates and equilibrium, calculations and organic chemistry
Find out more by working through a topic
- count4 of 9

- count5 of 9

- count6 of 9

- count7 of 9
