What are the key learning points about redox, rusting and iron?
Oxidation is the gain of oxygen, loss of hydrogen or loss of electrons. Reduction is the loss of oxygen, gain of hydrogen or gain of electrons. In a redox reaction, oxidation and reduction take place simultaneously.
Rust is formed when iron is exposed to water and air. There are several methods to prevent rust from forming.
Iron is extracted from haematite in the blast furnace. Reactions take place in the furnace to form carbon monoxide, which is the reducing agent that converts iron oxide into iron.
What are oxidation and reduction?
Oxidation and reduction describe two types of chemical reaction.
There are three different ways that a reaction could be described as oxidation or reduction.
| Oxidation | Reduction |
|---|---|
| Gain of oxygen | Loss of oxygen |
| Loss of hydrogen | Gain of hydrogen |
| Loss of electrons | Gain of electrons |
These examples show how to explain oxidation and reduction.
Often you can explain it in terms of change in oxygen content or hydrogen content but sometimes an explanation in terms of electrons is required.
1 . A change in oxygen content
Magnesium is oxidised when it reacts with oxygen to form magnesium oxide, according to the equation below:
2Mg + O2 → 2MgO
Explain, in terms of oxygen content, why this is an oxidation reaction.
Answer
Magnesium gains oxygen and the gain of oxygen is oxidation.
2. A change in hydrogen content
Nitrogen reacts with hydrogen to form ammonia according to the equation below:
N2 + 3H2 → 2NH3
Explain, in terms of hydrogen content, why nitrogen is described as being reduced.
Answer
Nitrogen gains hydrogen and the gain of hydrogen is reduction.
3. A change in electrons
Iron can react to form iron(iii) ions according to the equation below:
Fe → Fe3+ + 3e-
Explain, in terms of electrons, whether this is an oxidation or reduction reaction.
Answer
Iron loses electrons and the loss of electrons is oxidation.
What is redox?
A redox reaction is a reaction where oxidation and reduction take place simultaneously (at the same time).
What is an example of redox? (Higher tier only)
Magnesium reacts with copper(II) sulfate solution according to the equation:
Mg(s) + CuSO4(aq) → MgSO4(aq) → Cu(s)
Explain, in terms of electrons, why this reaction is a redox reaction.
Answer
The slideshow shows how to convert the equation in the question into two half equations.
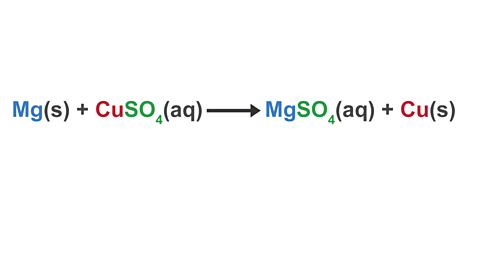
Image caption, 1. Start by splitting the ionic compounds in the equation into separate ions.
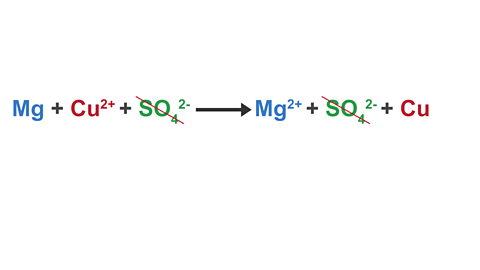
Image caption, 2. The SO₄²⁻ ions do not change in the reaction – they are spectator ions. These ions are removed from the equation.
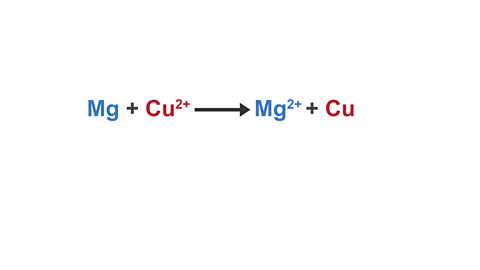
Image caption, 3. This is now an ionic equation. Mg and Cu can be separated into two half equations.
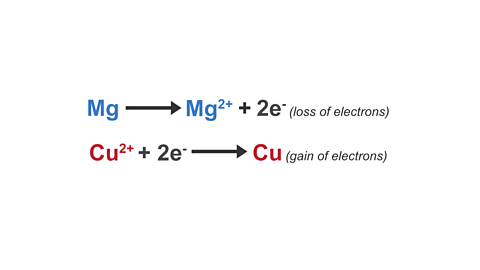
Image caption, 4. The two half equations can be used to explain why this reaction is a redox reaction.
1 of 4
Explanation
Magnesium loses electrons and loss of electrons is oxidation.
Copper(II) ions gain electrons and gain of electrons is reduction.
Redox is when oxidation and reduction occur simultaneously in the same reaction.
What is rusting?
Rusting is an oxidation reaction.
The iron reacts with water and oxygen to form hydrated iron(III) oxide, which is rust.
Here is the word equation for the reaction:
iron + water + oxygen → hydrated iron(III) oxide
Rust has the appearance of a red-brown flaky solid.
Iron and steel rust when they come into contact with water and oxygen – both are needed for rusting to occur.
In the experiment below, the nail does not rust when either air (containing oxygen) or water is not present:
Boiling the water removes dissolved oxygen and the layer of oil prevents it from re-entering.
Anhydrous calcium chloride removes water vapour from the air.
How to prevent rusting

There are several ways to prevent iron and steel rusting. Some work because they stop oxygen or water reaching the surface of the metal:
- oiling – for example, bicycle chains
- greasing – for example, nut and bolts
- painting – for example, car body panels
- coating with a thin layer of plastic

Iron and steel objects may also be covered with a layer of metal.
Food cans are plated with a thin layer of tin.
What is sacrificial protection?
Magnesium and zinc are often used as sacrificial metals.
They are more reactive than iron and react with water and air before the iron does.
This prevents the iron from rusting.
What is galvanising?
Galvanising is a method of rust prevention.
The iron or steel object is coated in a thin layer of zinc.
This stops oxygen and water reaching the metal underneath – but the zinc also acts as a sacrificial metal.
Zinc is more reactive than iron, so it reacts before the iron does.
How is iron extracted?
Iron is used in bridges, buildings and other structures because it is strong.
Iron is very useful, but it cannot be found in the ground as pure metal.
It must be extracted from iron ore (haematite).
What happens in a blast furnace?
Iron is extracted from iron ore in a huge container called a blast furnace.
Iron ores such as haematite contain iron(III) oxide, Fe2O3.
The oxygen must be removed from the iron(III) oxide to leave the iron behind.
What are the raw materials used in a blast furnace?
There are four raw materials that go into the blast furnace.
Iron ore, coke and limestone enter from the top and hot air is blasted in at the bottom.
| Raw material | Contains | Function |
|---|---|---|
| Iron ore (haematite) | Iron(III) oxide (Fe2O3) | A compound that contains iron |
| Coke | Carbon (C) | Burns in air to produce heat, and reacts to form carbon monoxide (needed to reduce the iron oxide) |
| Limestone | Calcium carbonate (CaCO3) | Helps to remove acidic impurities from the iron by reacting with them to form molten slag |
| Air | Oxygen (O2) | Allows the coke to burn, and so produces heat |
What reactions take place in a blast furnace?
In total, five reactions take place in the blast furnace:
Formation of carbon monoxide
The carbon (in coke) burns to form carbon dioxide
- carbon + oxygen → carbon dioxide
C(s) + O2(g) → CO2(g)
Carbon dioxide reacts with more carbon to form carbon monoxide.
- carbon dioxide + carbon → carbon monoxide
CO2(g) + C(s) → 2CO(g)
The carbon monoxide is the reducing agent which reduces iron(III) oxide to iron.
Reduction of iron(III) oxide
The carbon monoxide produced reacts with the iron(III) oxide in the haematite.
This produces liquid iron, and is an example of a reduction reaction as iron(III) oxide loses oxygen.
- iron(III) oxide + carbon monoxide → iron + carbon dioxide
Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2(g)
Molten iron is tapped off at the bottom of the blast furnace.
Removing impurities
Haematite contains silica impurities which would build up over time in the blast furnace if they were not removed.
The calcium carbonate in the limestone thermally decomposes to form calcium oxide.
- calcium carbonate → calcium oxide + carbon dioxide
CaCO3(s) → CaO(s) + CO2(g)
The calcium oxide then reacts with silica (sand) impurities in the haematite, to produce slag – which is calcium silicate.
- calcium oxide + silica → calcium silicate
CaO(s) + SiO2(s) → CaSiO3(l)
This reaction is a neutralisation reaction.
Calcium oxide is basic (as it is a metal oxide) and silica is acidic (as it is a non-metal oxide).
Molten slag is tapped off at the bottom of the blast furnace above the molten iron as molten slag is less dense than molten iron.
How much do you know about redox, rusting and iron?
More on Unit 2: Further chemical reactions, rates and equilibrium, calculations and organic chemistry
Find out more by working through a topic
- count3 of 9

- count4 of 9

- count5 of 9

- count6 of 9
